Sure, mitochondria, the nucleus, and the Golgi apparatus are cool, but I don’t understand why more people aren’t talking about cilia. Cilia are definitely in my top three favorite eukaryotic organelles for sure! Maybe this post will boost their ranking for you, too?

These look like some sort of coral at the bottom of the ocean, but it’s actually cilia from someone’s lungs… :\ (From Wikipedia)
Did you know that cilia exist on almost all mammalian cells??? Okay, I guess primary cilia can be super short and sometimes don’t even project past the cell surface (awkward), but they are still there, and they are not – as was previously assumed – vestigial! Still, motile cilia are the more highly publicized type of cilia. You might, for example, remember that the cilia lining your respiratory tract are important for pushing the dirty mucus out of your lungs. Gross/important.
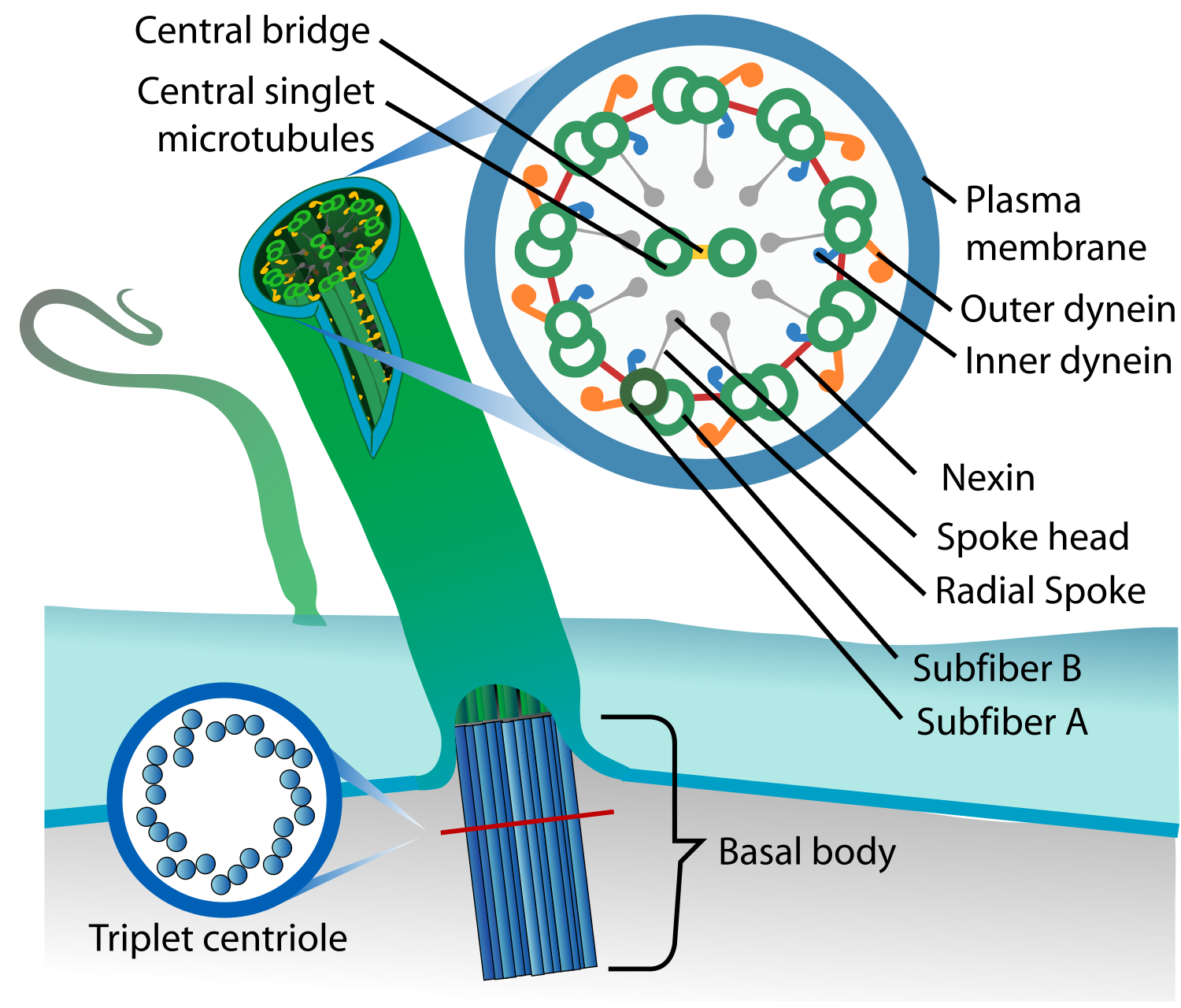
Cilia aren’t just hollow tubes! (From Wikipedia)
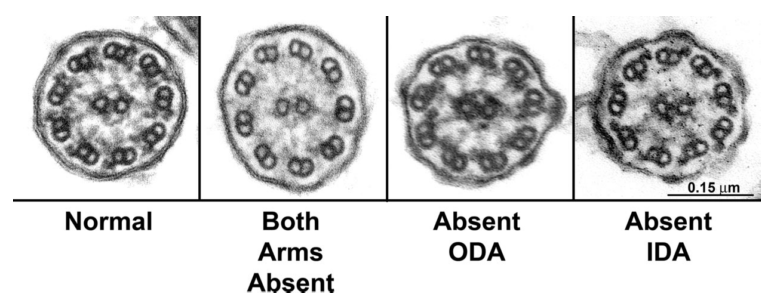
Cilia structure is complex. All cilia have a cytoskeletal “axoneme” composed of 9 pairs of microtubules. These microtubule doublets form a cylinder. If the cylinder surrounds two additional microtubules, you’ve got a motile “9+2” axoneme. If the cylinder is empty, you’ve got a primary “9+0” axoneme. The outer microtubule doublets often have inner dynein arms (IDAs, in blue above) and outer dynein arms (ODAs, in orange above) that act as motors to get the cilia moving.
Generally, motile cilia beat at a frequency of 8-20 Hz.1 Sometimes, though, genetic mutations cause some cilia to malfunction, thus producing asynchronous or weak beats. This condition is called primary ciliary dyskinesia, or PCD.
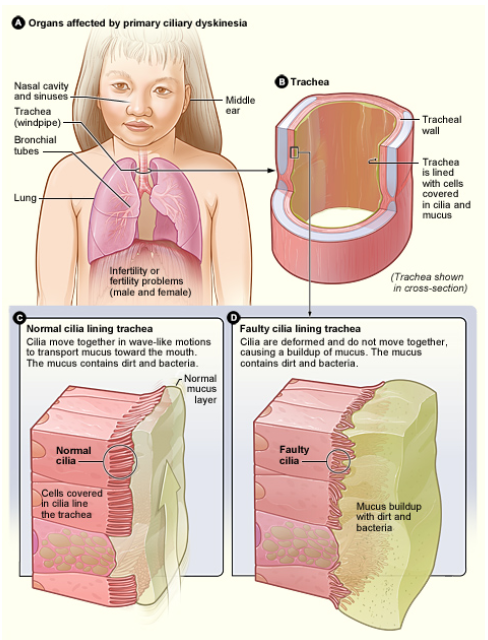
Learn more about PCD from the NIH National Heart, Lung, and Blood Institute!
PCD is a highly heterogeneous condition that occurs once in every 16,000 births. Common symptoms include chronic bronchitis, sinusitis, otitis media, and reduced male fertility (sperm flagella share the same structures as 9+2 cilia). 50% of patients with PCD have laterality defects, situs inversus or situs ambiguous, which means their internal organs are on the completely opposite side of the body or, worse, somewhere in the middle.2
PCD can be caused by a number of mutations, most commonly those that affect ODA structure and function.3 12% of PCD cases occur due to improper 9+2 and IDA structure. Of these, over half are due to mutations in our gene-of-the-week coiled-coil domain containing 40 gene (CCDC40), related CCDC39, or both!4 CCDC40 is expressed in the embryonic node and midline, both important for normal lateralization. The gene is also important for the normal recruitment and function of DNALI1 and GAS8/11, which are necessary for the assembly of IDAs and dynein regulatory complexes, respectively. The exact mechanisms by which CCDC40 acts in lateralization and axoneme formation, however, are still not understood.
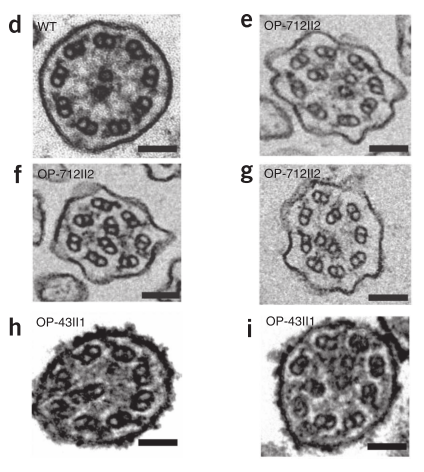
Transmission electron microscopy of respiratory cilia in a control (D) and two PCD patients (OP-712II2 and OP-43II1, E-I). From Becker-Heck et al., 2011.
PCD patients with CCDC40 mutations show absent, extra, or eccentric (misplaced) central microtubules. They may also show displaced outer doublets, reduced IDAs, and abnormalities in other ciliary structures (such as radial spokes and nexin links).5
In mice, mutated Ccdc40 causes situs inversus (8%) and left isomerism (19%).

Mutations in mouse Ccdc40. A. Normal situs of an E13.5 wildtype mouse. B. left isomerism of an E13.5 mutant mouse. C. Normal situs of an E12.5 wildtype mouse. D. Situs inversus of an E12.5 mutant mouse. RV: right ventricle, R: right lung lobe, L: left lung lobe, S: stomach. From Becker-Heck et al., 2011.
In zebrafish, ccdc40 mutations result in disrupted laterality and the “curly-tail” phenotype.

Mutations in zebrafish ccdc40. E. Normal zebrafish embryo. F. Mutant ccdc40 embryo with the curly-tail phenotype. From Becker-Heck et al., 2011.
CCDC40 mutations across species cause the reduction of amplitude or complete immotility of 9+2 cilia. This results in asynchrony of movement and a decrease in mean ciliary beat frequency (to <3 Hz!).67 These funky beats are not fresh. Fortunately, however, this mutation leaves ODAs intact, so at least motility is partially retained, and permanent treatments for PCD (rather than constant antibiotics and transplants) are being developed.
https://www.youtube.com/watch?v=Uvs5cOJYw7U
So anyway, be thankful for your cilia. Aren’t they the coolest?
There have been no recent publications on CCDC40, but they may be in the works. At the end of July, Jorge and Leslie Bacardi donated a substantial (undisclosed) sum of money to the Mayo Clinic in Jacksonville, Florida. Jorge Bacardi suffers from PCD and received a lung transplant from the Lung Transplant Program at the Mayo Clinic in 2008!
References:
- Leigh, M.W., Pittman, J.E., Carson, J.L., Ferkol, T.W., Dell, S.D., Davis, S.D., Knowles, M.R., and Zariwala, M.A. (2009). Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 11, 473–487. [↩]
- Leigh, M.W., Pittman, J.E., Carson, J.L., Ferkol, T.W., Dell, S.D., Davis, S.D., Knowles, M.R., and Zariwala, M.A. (2009). Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 11, 473–487. [↩]
- Antony, D., Becker-Heck, A., Zariwala, M. a, Schmidts, M., Onoufriadis, A., Forouhan, M., Wilson, R., Taylor-Cox, T., Dewar, A., Jackson, C., et al. (2013). Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 34, 462–472. [↩]
- Antony, D., Becker-Heck, A., Zariwala, M. a, Schmidts, M., Onoufriadis, A., Forouhan, M., Wilson, R., Taylor-Cox, T., Dewar, A., Jackson, C., et al. (2013). Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat. 34, 462–472. [↩]
- Becker-Heck, A., Zohn, I.E., Okabe, N., Pollock, A., Lenhart, K.B., Sullivan-Brown, J., McSheene, J., Loges, N.T., Olbrich, H., Haeffner, K., et al. (2011). The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 43, 79–84. [↩]
- Leigh, M.W., Pittman, J.E., Carson, J.L., Ferkol, T.W., Dell, S.D., Davis, S.D., Knowles, M.R., and Zariwala, M.A. (2009). Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet. Med. 11, 473–487. [↩]
- Chilvers, M., Rutman, A., and O’Callaghan, C. (2003). Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunology 112, 518–524. [↩]


Trackbacks/Pingbacks